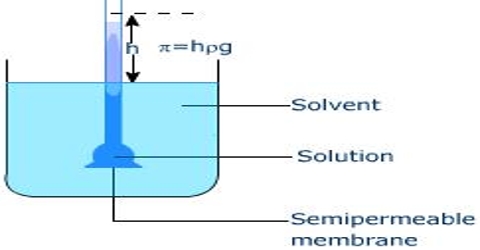
Osmotic pressure- Berkley Hartley’s method

source : Self Study Point
Osmotic pressure by Berkley Hartley’s method-
Principle-
The principle of Berkley Hartley’s method is to apply external pressure on the solution by applying weight on the piston , just sufficient to prevent the osmosis of the solvent into it.
Method-

source : meritnation
The apparatus consists of a porous pot containing Cu- ferrocyanide deposited on its wall. Cu- ferrocyanide acts as a semipermeable membrane (SPM). It is fitted into a bronze cylinder to which is fitted a piston and a pressure gauze . The function of pressure gauze is to measure the pressure . The porous pot is fitted with a water reservoir on one side and a capillary tube on the other side .
Procedure-
Water is kept in the porous cylinder while bronze cylinder is filled with solution ( whose osmotic pressure is to be measured).Water placed in the porous cylinder tends to pass into solution through semipermeable membrane then level in the capillary tube moves downward, then external pressure is applied on the piston so that water level in the capillary tube remains constant.The reading of pressure gauze is recorded. This applied pressure is equal to the osmotic pressure.
Conditions-
1) The solute must be non volatile.
2) The solute should not undergo either dissociation or association in the solution.
3) The solution must be dilute (not more than 5 %)
Advantage-
1) It is quick and accurate method.
2) This method can be used to measure high osmotic pressure also because osmotic pressure is balanced by external pressure, there is no strain on semipermeable membrane and no danger of its bursting.
3) The results obtained by this method are reliable because the concentration of solution does not change because flow of the solvent is not permitted in the solution.
Reverse Osmosis-
The process of movement of solvent through a semipermeable membrane from the solution to the pure solvent by applying excess pressure on the solution is called ‘Reverse Osmosis’.
Ex- Desalination of sea water-
In this process , pressure greater than osmotic pressure is applied on the sea water(this is equivalent to solution). The pure water flows from the salt water to pure water through semipermeable membrane . Thus pure water is obtained which does not contain undesirable salts.

source : angelwater.com