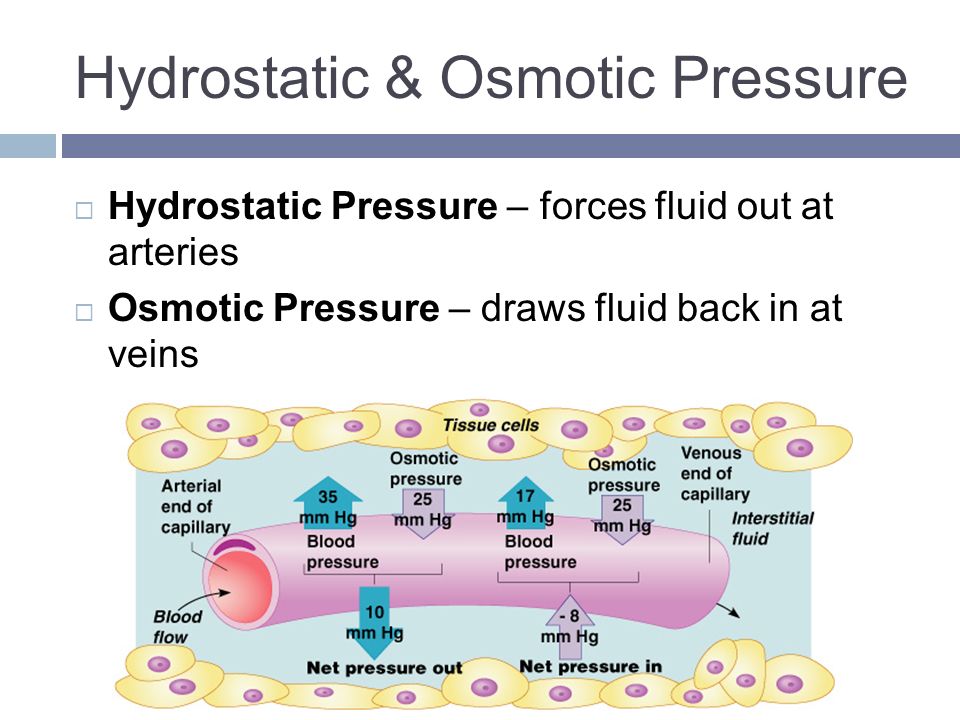
Osmotic pressure –

source : study.com
Osmotic pressure-
Question 1) A 0.1 M solution of potassium ferrocyanide is 46 % dissociated at 18 0 C. Calculate osmotic pressure of the solution ?
Solution )
0.1 M means 0.1 mole of solute in 1 litre of solution.
π = ? atm , S = 0.0821 litre atm K -1mole-1 , T = 18 0 C = 273 + 18 =291 K , n = 0.1 mole , V = 1 litre
π N = n ST / V
π N = 0.1 x 0.0821 x 291 / 1
π N= 2.389 atm.
K4 [Fe (CN)6] ——–> 4 K + + [Fe (CN)6] 4-
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ 4 ∝ ∝
Total moles after dissociation = 1 – ∝ + 4∝ + ∝ = 1 + 4∝
% of ∝ = 46 % , ∝ = 46 / 100 = 0.46
π exp / π N = (1 + 4 x 0.46 ) / 1
π exp / 2.389 = ( 1 + 4 x 0.46 )
π exp = 2.389 x 2.84
π exp = 6.78 atm Ans.
Question 2) Calculate the freezing of an aqueous solution of a nonvolatile , nonelectrolyte solute having an osmotic pressure of 2.0 atm at 300 K .
( Kf = 1.86 K Kg mole-1 , S = 0.0821 Litre atm K-1 mole-1)
Solution )
C (molarity ) = ?
T =300 K , S = 0.0821 Litre atm K-1 mole-1,
π = 2.0 atm
π = CST
C = 2.0 / 0.0821 x 300 = 0.0812 mole / litre
In dilute solution , the density of water = 1 gm / cc
ΔTf = Kf x molality
ΔTf = 1.86 x 0.0812
ΔTf = 0.151
freezing point of solvent (water ) = 0 + 273 = 273 K
ΔTf = freezing point of solvent – freezing point of solution
freezing point of solution = 273 – 0.151
freezing point of solution = 272.749 K
OR
freezing point of solvent (water ) = 00 C
ΔTf = freezing point of solvent – freezing point of solution
freezing point of solution = 0 – 0.151
freezing point of solution = – 0.151 0C Ans.
Question 3) The osmotic pressure of a dilute aqueous solution of a compound ‘X ‘ containing 0.12 gm / litre is twice the osmotic pressure of a dilute aqueous solution of another compound ‘Y’ containing 0.18 gm / litre .What is ratio of molecular weight of ‘X ‘
to that of ‘Y’ ? Both ‘X’ and ‘Y’ remain in the molecular form in solution .
Solution )
For ‘X’ ,
w1 = 0.12 gm , v1 = 1 litre
π1 = w1 S T / m1 v1
π 1 = 0.12 S T / m1 x 1
For ‘Y’ ,
w2 =0.18 gm , v2 = 1 litre
π2 = w2 S T / m2 v2
π2 = 0.18 ST /m2 x 1
π 1 /π 2 = 0.12 m2 / 0.18 m1
2 / 1 = 0.12 m2 / 0.18 m1
m2 / m1= 2 x 0.18 / 0.12
m2 / m1 = 3 /1
m1 : m2 = 1 : 3 Ans.
Question 4 ) A decinormal solution of NaCl is 80 % dissociated at 270 C. Calculate osmotic pressure of solution ?
( S = 0.0821 Litre atm K-1 mole-1 )
Solution )
π = ? atm , S = 0.0821 litre atm K -1mole-1 , T = 270 C = 273 + 27 = 300 K ,
Molecular weight of NaCl =23 + 35.5 = 58.5
Equivalent weight of NaCl = Molecular weight / total positive or negative valency
total positive valency = 1 (because Na+)
Equivalent weight of NaCl = 58.5 / 1 = 58.5
Molarity / normality = Equivalent weight/ molecular weight
M / N = 58.5 / 58.5
N = M
Decimolar solution means , M = 0.1,
Therefore, N =0.1
C is molarity of solution , C =0.1,
π N = C ST
π N = 0.1 x 0.0821 x 300= 2.46 atm
π N= 2.46 atm.
NaCl ——–> Na+ + Cl –
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ ∝ ∝
Total moles after dissociation = 1 – ∝ + ∝ + ∝ = 1 + ∝
% of ∝ = 80 % , ∝ = 80 / 100 = 0.80
π exp / π N = (1 + 0.80) / 1
π exp / 2.46 = 1.80
π exp = 2.46 x 1.80
π exp = 4.43 atm Ans.