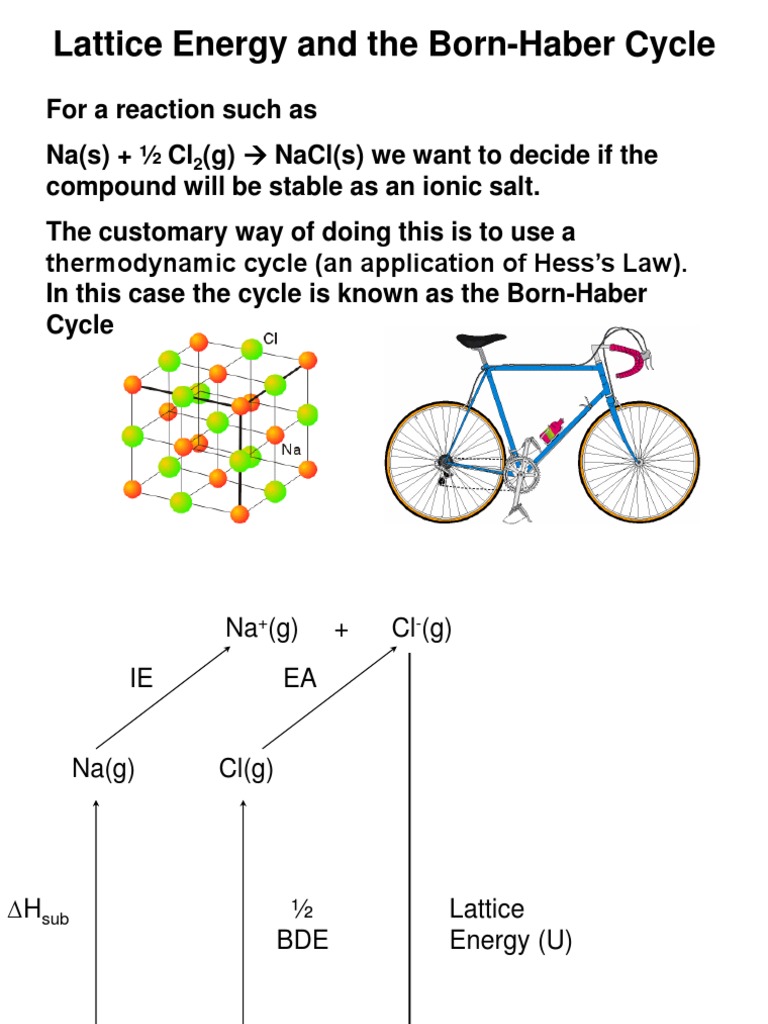
Born – Haber cycle

source : slideshare.net
Born – Haber cycle-
Question 1) – Calculate the lattice enthalpy of KCl from the following data at standard states-
Enthalpy of sublimation of K = 89 KJ/mole
Enthalpy of dissociation of chlorine = 244 KJ/ mole
Ionisation energy of K = 425 KJ/mole
Electron gain enthalpy of chlorine = -355 KJ/mole
Enthalpy of formation of KCl = -438 KJ/mole
Solution )
K(s) + ΔH sub ———> K(g) ; ΔH sub = 89 KJ/mole
1/2 Cl2 (g) + D/2 ——-> Cl(g) ; D/2 = 244/2 = 122 KJ/mole
K(g) + IE ——–> K+ (g) + e– ; IE= 425 KJ/mole
Cl(g) + e ———–> Cl– (g) +EA ; EA = -355 KJ/mole
K+ (g) + Cl– (g) ———> KCl (s) ; Δ H f 0 = -438 KJ/mole
According to Born – Haber cycle-
Δ H f 0 = ΔH sub + D/2 + IE + EA +U
-438 = 89 + 122 + 425 – 355 + U
U = – 719 KJ/mole
Lattice energy of KCl = 719 KJ/mole Ans.
Question 2) – Calculate the lattice enthalpy of CaCl2 , given that the enthalpy of –
Enthalpy of sublimation for Ca (s) —-> Ca(g) = 121 KJ/mole
Enthalpy of dissociation of Cl2 (g) —-> 2Cl(g) = 242.8 KJ/ mole
Ionisation energy of Ca(g) —–>Ca++ = 2422 KJ/mole
Electron gain enthalpy of 2Cl —–> 2 Cl– = 2 x -355 = -710 KJ/mole
Enthalpy of formation of CaCl2= -795 KJ/mole
Solution )
Ca (s) + ΔH sub ———> Ca (g) ; ΔH sub = 121 KJ/mole
Cl2 (g) + D ——-> 2Cl(g) ; D = 242.8KJ/mol
Ca(g) + IE ——–> Ca++ (g) + 2e– ; IE= 2422 KJ/mole
2Cl(g) +2 e ———–> 2Cl– (g) +EA ; EA = – 2x 355 = 710 KJ/mole
Δ H f 0 = -795KJ/mole
Ca++ (g) + 2Cl– (g) ———> CaCl2 (s)
According to Born – Haber cycle-
Δ H f 0 = ΔH sub + D + IE + EA +U
-795 = 121 + 242.8 + 2422 – 710 + U
U = – 2870.8 KJ/mole
Lattice energy of CaCl2 = 2870.8 KJ/mole Ans.
Question 3) Calculate the enthalpy of formation of MgF2 from the following data-
Enthalpy of sublimation of Mg = 146.4 KJ/mole
Enthalpy of dissociation of Fluorine = 155.8 KJ/ mole
Ionisation energy of Mg (IE2 ) = 2186.0 KJ/mole
Electron gain enthalpy of Fluorine = -322.6 KJ/mole
Lattice Enthalpy of MgF2 = – 2922.5 KJ/mole
Solution )
The heat of formation , Δ H f 0 may be expressed as,
Δ H f 0 = ΔH sub + D + IE + EA + U
Δ H f 0 = 146.4 + 155.8 + 2186 – 2 x 332.6 – 2922.5
Δ H f 0= 1099.5 KJ/mole Ans.