Physical Chemistry

Heat of combustion & formation
Heat of combustion & formation- Heat of combustion – “It is defined as the change in internal energy Continue Reading »

Thermodynamics
Thermodynamics- Thermodynamics – ‘The branch of science that deals with the study of different forms of energy & their Continue Reading »
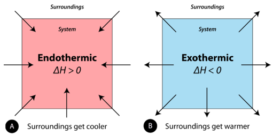
Enthalpy change
Enthalpy change- Enthalpy change- It is defined as the change in enthalpy or change in internal energy or amount Continue Reading »
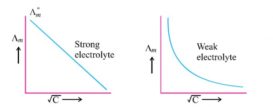
Kohlrausch law- Applications
Kohlrausch law- source : slideplayer.com Applications of Kohlrausch law- 1) Calculation of degree of ionisation and ionisation constant- Degree Continue Reading »

Find the solubility of MnS in neutral water in gm/l .Ksp of MnS = 2.6 x 10-14
Find the solubility of MnS in neutral water in gm/l ? Ksp of MnS = 2.6 x 10-14. Solution Continue Reading »
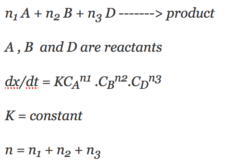
If the initial concentration of cyclopropane is 0.0501 M. What will the cyclopropane concentration after 352minutes.The reaction is first order. Rate constant = 3.36 x 10-5 sec-1.
Question – If the initial concentration of cyclopropane is 0.0501 M. What will the cyclopropane concentration after 352 minutes. Continue Reading »
What is the concentration of KMnO4 solution in mole/litre which gives an absorbance of 0.120 when measured in a 2.0 cm cell ?
Question – What is the concentration of KMnO4 solution in mole/litre which gives an absorbance of 0.120 when measured Continue Reading »
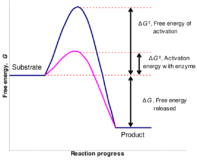
The rate constant for a reaction was measured as a function of temperature. A plot of lnK versus T ( in kelvin ) is linear and has a slope.Find the activation energy ?
Q – The rate constant for a reaction was measured as a function of temperature. A plot of lnK Continue Reading »
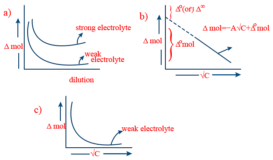
Kohlrausch law
Kohlrausch law source : AskIITians Kohlrausch law- According to this law, ” At infinite dilution , when ionisation is Continue Reading »
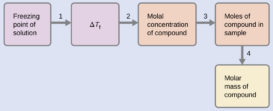
Depression in freezing point-Part 2
Depression in freezing point source : youtube.com Definition of Molecular or molar depression constant- If w/m = 1 mole, Continue Reading »