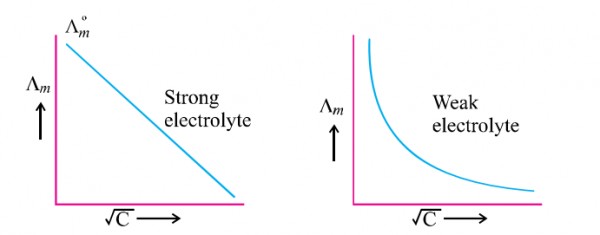
Kohlrausch law-

source : slideplayer.com
Applications of Kohlrausch law-
1) Calculation of degree of ionisation and ionisation constant-
Degree of ionisation ‘∝’ = Λm / Λ0m
Λm = molar conductance at any conc.
Λ0m = molar conductance at infinite dilution
∝ = Λeq / Λ0eq
ionisation constant= C ∝2 /(1 -∝)
2) Calculation of solubility product of sparingly soluble salt-
Λeq = K x 1000 /C
C = conc. of solution or molarity
Since solution is very dilute so,
Λeq = Λ0eq
Λ0eq = K x 1000 / C
We can calculate solubility of sparingly soluble salt by this formula.
C = solubility of salt
Λ0eq can be calculated by Kohlrausch law,
Λ+eq = Λ0+ + Λ0–
3) Calculation of equivalent or molar conductance of weak electrolyte at infinite dilution-
Weak electrolytes are not completely ionises at any dilution therefore their equivalent or molar conductance can not be determine experimentely . So their molar or equivalent conductance at infinite dilution can be calculated by Kohlrausch law.
Ex 1) Molar conductivity of CH3COOH can be calculated by Kohlrausch law,
Λ+CH3COOH = Λ0CH3COO– + Λ0H+————eq1
The above eq. can be obtained by knowing the molar conductivity at infinite dilution for strong electrolyte like sodium acetate-
Λ+CH3COONa = Λ0CH3COO– + Λ0Na+————eq2
Λ+HCl = Λ0H+ + Λ0Cl– ————eq3
Λ+NaCl = Λ0Cl– + Λ0Na+————eq4
Adding eq (2) and (3) and subtracting eq(4)
Λ+CH3COONa + Λ+HCl – Λ+NaCl = Λ0CH3COO– + Λ0Na+ + Λ0H+ + Λ0Cl– – Λ0Cl– – Λ0Na+
Λ+CH3COONa + Λ+HCl – Λ+NaCl = Λ0CH3COO– + Λ0H+
Ex 2) Molar conductivity of NH4OH can also be calculated by Kohlrausch law-
Λ+NH4OH = Λ0 NH4 + + Λ0OH– ———-eq 1
The above eq. can be obtained by the knowing the molar conductivities at infinite dilution for strong electrolyte NH4Cl, NaOH , NaCl.
Λ+NH4OH = Λ0OH– + Λ0NH4+————eq1
The above eq. can be obtained by the knowing of molar conductivity at infinite dilution for strong electrolyte like NH4Cl,NaOH,NaCl.
Λ+NH4Cl = Λ0Cl– + Λ0NH4+————eq2
Λ+NaOH = Λ0Na+ + Λ0OH– ————eq3
Λ+NaCl = Λ0Cl– + Λ0Na+————eq4
Adding eq (2) and (3) and subtracting eq(4)
Λ+NH4Cl + Λ+NaOH – Λ+NaCl = Λ0Cl– + Λ0NH4+ + Λ0Na+ + Λ0OH– – Λ0Cl– – Λ0Na+
Λ+NH4Cl + Λ+NaOH – Λ+NaCl = Λ0 OH– + Λ0NH4+