Molecular orbital

source : saylordot org.github.io
Molecular Orbital Theory –
This theory was suggested by F. Hund and R.S Mulliken and developed later by Lennard-Jones and Charles Coulson and others.
Orbital of an electron in a molecule is called molecular orbital. In a molecule an electron spends most of the time in its molecular orbital. Thus the probability of finding the electron is highest in its molecular orbital . A molecular orbital can accomodate maximum two electrons of opposite spin.
In atomic orbital electron is under the influence of one nucleus only. In molecular orbital electron is under the influence of two or more nuclei .
Linear combination of atomic orbitals (LCAO) :
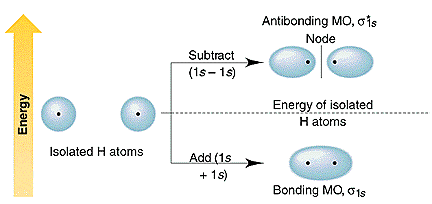
Two atomic orbitals overlap to give two molecular orbitals (M.O) . Hence number of M.O is equal to the total number of atomic orbitals taking part in overlapping . There are two types of molecular orbital.
a) Bonding M.O :
A bonding M.O (φb) is formed when atomic orbitals φ1 and φ2 are added or the two atomic orbitals are in one phase and overlap.
φb = φ1 + φ2
The energy of bonding M.O is lower than the energy of atomic orbitals. The probability of finding electron in bonding M.O is high. Bonding M.O is represented by σ , π and δ.
b) Antibonding M.O :
An anti bonding M.O (φa) is formed by subtracting the atomic orbitals (φ1 and φ2) .
φa = φ1- φ2
The energy of anti bonding M.O is higher than the energy of atomic orbitals. The anti bonding orbitals are represented by σ*, π* and δ* etc.

source : Slide player
Energy of Molecular Orbital –
a) Energy of all the molecular orbitals whether bonding or anti bonding is not equal. Their energy increases in the following order.
Number of electrons > 14
σ1s < σ*1s<σ2s<σ*2s <σ2px< π2py = π2pz < π*2py = π*2pz < σ*2px
b) Number of electrons < or = 14, their energy increases in the following order:
σ 1s< σ*1s<σ2s<σ*2s < π2py = π2pz <σ2px <π*2py = π*2pz < σ*2px
c) Energy of π2py and π2pz molecular orbitals is equal , similarly energy of π*2py and π*2pz orbitals is equal.
d) The maximum number of electrons in a M.O is two and these two electrons must be of opposite spin (Pauli’s exclusion principle)
e) If there are two molecular orbitals of the same energy, pairing of electrons take place only after each orbitals of the same energy has one electron (Hund’s rule)
f) Electrons are filled in these orbitals according to Aufbau principle. The molecular orbitals of lowest energy are filled first.
After filling two electrons in σ 1s molecular orbital, filling of σ*1s orbital starts. Similarly after filling two electrons in σ 2s molecular orbital , filling of σ*2s orbital starts. One electron each in π2py and π2pz is filled first and then pairing begins in these molecular orbitals similarly in π*2py and π*2pz.

source : chem.libre texts.org