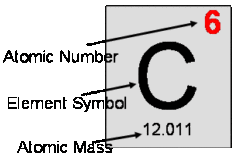
Atomic mass
Atomic mass source: Difference between Atomic mass- ” Atomic mass of an element can be defined as the number Continue Reading »

Schrodinger wave equation
Schrodinger wave equation source : transtutors.com Schrodinger wave equation- In quantum mechanics , the Schrodinger wave equation is a Continue Reading »
Solubility product Numerical part 3
Solubility product source : toppr Solubility product Numerical- Question 1) The solubility of Mg(OH)2 in a particular buffer is Continue Reading »

Solubility product- Numerical part 1
Solubility product source: webstockreview.net Solubility product- Numerical Question 1) The solubility product of AgCl is 1.5625 x 10 -10 Continue Reading »
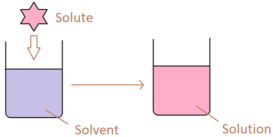
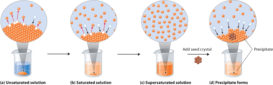
Solubility product
Solubility product source : Chemistry Libre Texts Solubility product- A solution which remains in contact with undissolved solute is Continue Reading »

Abnormal colligative properties
Abnormal colligative properties- source : Emaze Abnormal colligative properties- Dilute solutions containing non volatile solute exhibits some Continue Reading »
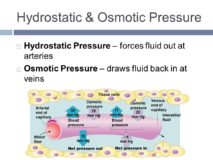
Osmotic pressure Numerical Part 3
Osmotic pressure – source : study.com Osmotic pressure- Question 1) A 0.1 M solution of potassium ferrocyanide is 46 % Continue Reading »
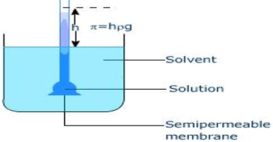
Osmotic pressure- Berkley Hartley’s method
Osmotic pressure- Berkley Hartley’s method source : Self Study Point Osmotic pressure by Berkley Hartley’s method- Principle- The principle Continue Reading »
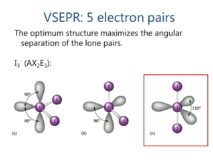
VSEPR Theory -applications part 3
VSEPR Theory source : Slideplayer.com VSEPR Theory [Valence Shell Electron Pair Repulsion Theory] -Applications- 1) Molecules containing five electron pairs- Continue Reading »
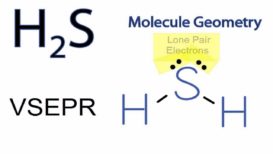
VSEPR Theory-Applications part 2
VSEPR Theory source : Slideserve.com VSEPR Theory [Valence Shell Electron Pair Repulsion Theory] -Applications- Shapes of molecules containing lone Continue Reading »